It is extremely costly and labour intensive for Manufacturers to manage medical device and drug safety recalls. These cost approximately 100k and often yield very little feedback with poor outcome data.
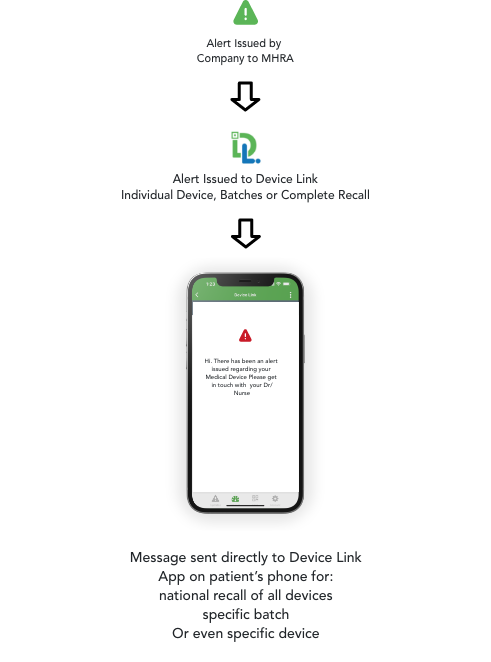
Device Link supports Manufacturers with their regulatory obligations for Post Market Surveillance and Post Market Clinical Follow-Up processes. It facilitates end-user notification to both Patients and Healthcare Professionals alike, offering a more cost effective, real-time agile solution to the problem of managing product alerts. Capability to capture real world product feedback from end users.
Device Link is already being actively used within the NHS.
Device Link supports Manufacturers with device demographic data and feedback alongside Patient and Public engagement.